A ‘groundbreaking’ drug to slow down the development of type 1 diabetes is being tried by a handful of patients in the UK.
Teplizumab, which is already approved in the US, trains the immune system to stop attacking pancreatic cells, delaying the need for insulin by an average of three years.
With type 1 diabetes, the immune system attacks insulin-producing cells in the pancreas, meaning blood sugar levels are no longer regulated by the body.
If blood sugar is too high or low, it can cause serious health problems and even death.
People with type 1 therefore need daily insulin.
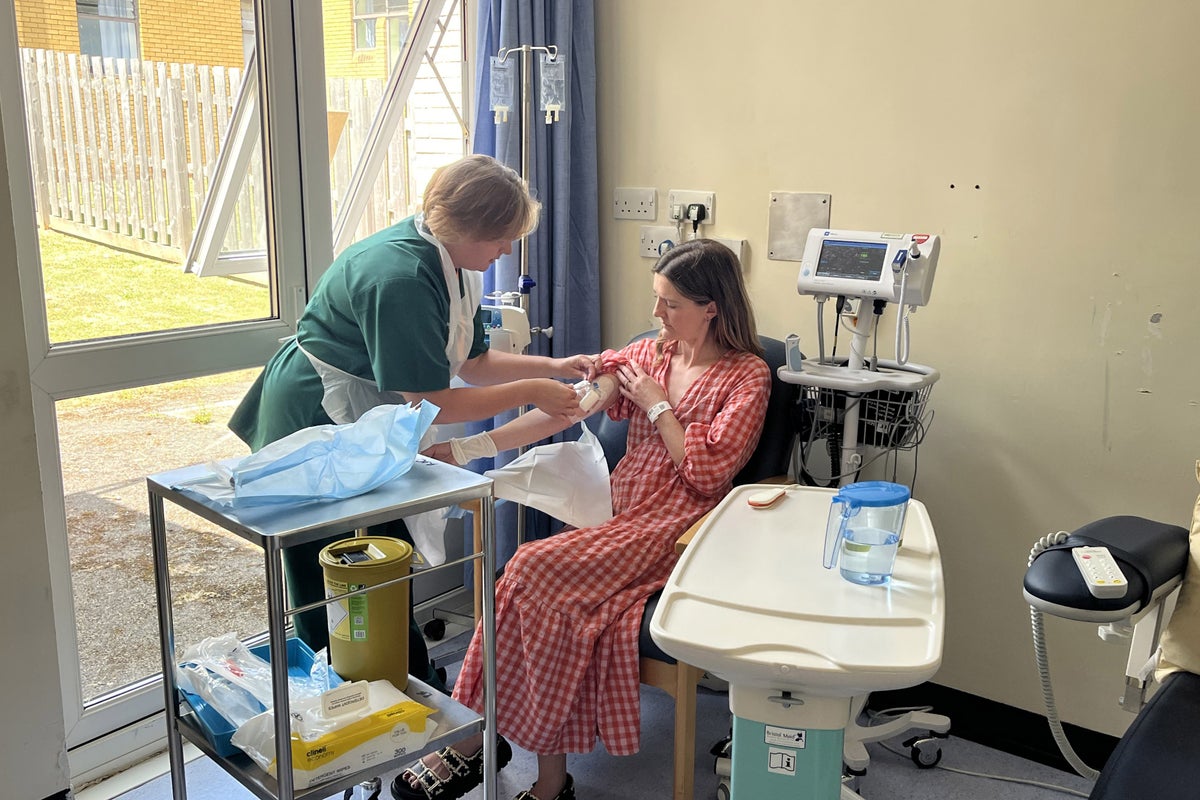
Dentist and mother-of-two, Hannah Robinson, from Devon, is the first adult in the UK to try the drug in the hope it can delay the condition.
She is having treatment at the Royal Devon University Healthcare NHS Foundation Trust after discovering during pregnancy she was in the early stages of developing type 1.
A handful of people are being given the medicine on a case-by-case basis while it is reviewed for wider use on the NHS.
The 36-year-old said: “For me, this new drug offers more freedom and the chance to focus on my health before I have to start thinking differently and managing life as somebody needing daily insulin.
“This isn’t just about what I eat or monitoring my glucose, it is also about having more control and not feeling defined by my condition.
“This treatment could potentially pave the way for a future cure for type 1 diabetes, which is incredible. I feel very lucky to be part of this.”
The new drug teplizumab must be given at the earliest stage of the disease to be effective.
Dr Nick Thomas, diabetes consultant and academic clinical lecturer at the University of Exeter, said: “This new treatment represents a really exciting shift in how we manage type 1 diabetes.
“For the first time ever, we will be able to provide targeted treatment early enough in the process to alter the underlying immune process, aiming to slow down how quickly people need insulin.
“Approximately half of all type 1 diabetes cases develop in adulthood, and Hannah will be the first adult in the UK to receive this treatment.
“My hope is that in the future, we may be able to stop people with early type 1 diabetes from needing insulin at all.”
Experts at the Royal Devon and the University of Exeter are using genetics and other testing to spot people at high risk of developing type 1 diabetes.
The hope is more people could be offered the drug to delay type 1.
Dr Lucy Chambers, head of research impact and communications at Diabetes UK, said: “For people in the early stages of type 1 diabetes, teplizumab offers a groundbreaking opportunity to buy them precious extra years insulin-free.
“Right now, it’s only available in research settings – and while the excitement is real, urgent work is still needed to ensure it reaches everyone who could benefit.
“That means securing a UK licence for teplizumab, establishing national screening programmes to identify people with early-stage type 1 diabetes before symptoms appear, and preparing the NHS to deliver this treatment at scale.
“Diabetes UK is proud to be at the forefront of these efforts – funding pioneering research and working closely with the NHS towards a future where immunotherapies become the first-line treatment for tackling the autoimmune attack at the root of type 1 diabetes.”
Professor Richard Oram, consultant physician at the Royal Devon and professor at the University of Exeter, said: “Excitingly, teplizumab is the first drug with the potential to delay type 1 diabetes, but needs to be given before clinical diagnosis due to high blood glucose.
“It is really important to find new and improved approaches for identifying individuals at elevated risk.”
